감염생물학 연구실
연구실 정보
- 담당교수 : 양철수 교수
- 홈페이지 : http://infection8137.wixsite.com/chulsuyang
- 전화번호 : 031-436-8137
- 연구실 위치 : 제1과학기술관 5층 520호
연구실 소개내용
- 감염생물학연구실에서는 병원 미생물학 및 면역학 개념을 기반으로 한 ① 기초 연구와 ② 응용 연구를 수행하고 있습니다.
- ① 기초 연구 : 신/변종 출몰 미생물들의 병인 기전 연구를 통한 미생물-숙주 세포 상호 작용 이해
- - 결핵균, 리스테리아균, 캔디다 진균, 클라미디아균, 기생충 톡소포자충
- - 감염/염증 신호 조절의 미토콘드리아 역할 및 후성 유전학적 연구
- ② 응용 연구 : 미생물들의 숙주 세포 면역계 회피 기전을 응용한 감염/염증 및 종양 치료/제어 기반 기술 개발 연구
- - 감염 질환, 패혈증, 류마티스 관절염, 염증성 대장염, 다양한 종양
- 활성 산소 조절, 세포 자살 및 자가 포식 기전 연구 통한 염증 질환 치료
Research Interests
- 1. Molecular and cellular pathogenesis of intracellular microbes infection especially on Mycobacteria, Listeria, Candida, Toxoplasma, Influenza infection
- 2. Signal transduction mechanisms in immune and inflammatory responses
- 3. Host-pathogen functional interactions
- 4. Molecular and immunopathogenesis in neuroinflammatory diseases
- 5. The role of ROS-generating NADPH oxidases activation and expression
- 6. Exploring of cell death and autophagy pathways in microbial infection
- 7. The role of nuclear receptors in innate immunity
- 8. The role of mitochondria and autophagy in innate immunity
Research Statements
Mycobacterium tuberculosis : Mycobacterium tuberculosis (Mtb) is a major intracellular pathogen of humans, with an estimated one-third of the global population being latently infected, 2–3 million deaths annually from tuberculosis (TB), and an increasing number of multidrug-resistant TB case. My research interest lies in detailing Mtb infection-phagocytosis-autophagy connection to understand the crosslink of two ancient innate immune mechanisms and design novel microbicidal strategy against Mtb infection.
Host-Pathogen Interaction in Mycobacterial Infection : After exposure to Mtb, only 5-10% exposed individuals are known to develop tuberculosis. Thus the appropriate induction of host protective immune responses is crucial for antimicrobial resistance to mycobacteria. However, pathogenic mycobacteria can resist phagosomal maturation in the infected host cells, and have evolved complicate strategies to escape from host innate and adaptive immune responses. Dr. Yang’s main interest in his research is an elucidation of cross-talks in pathogen-host cell interface. Understanding of detailed host-pathogen interaction in mycobacterial infection will contribute to develop new vaccines and therapeutics against tuberculosis.
Autophagy and Phagocytosis : Phagocytes such as neutrophils and monocytes play an essential role in host defenses against microbial pathogens. Specifically, the phagocytic NADPH oxidase is recognized as a critical component of this innate immunity, responsible for the generation of microbicidal reactive oxygen species (ROS). Additionally, autophagy is a cytoprotective mechanism involving the formation of double-membrane vesicles, called autophagosomes, which sequester cytoplasmic damaged organelles, protein aggregates, or invading pathogens for degradation. Autophagy also couples and facilitates the oxidative and nonoxidative killing activities of the phagocytic NADPH oxidase, but the detailed mechanism of this cooperation remains elusive. My research interest lies in elucidating the crosstalk between phagocytosis and autophgay to build up the effective intracellular innate immune milieu against microbial pathogens.
Molecular Mechanisms of Innate Immune Responses : During last several years, Dr. Yang has focused on the investigations of molecular mechanisms in innate immune signaling, i.e., Toll-like receptors- and Dectin-1-dependent pathways, in phagocytes. Elucidating the mechanisms involved in the mycobacteria-mediated innate signaling pathways is the main topic of research in Dr. Yang. Other topics include the molecular mechanism of cross-talk between ROS-dependent and host defense signal transduction pathways.
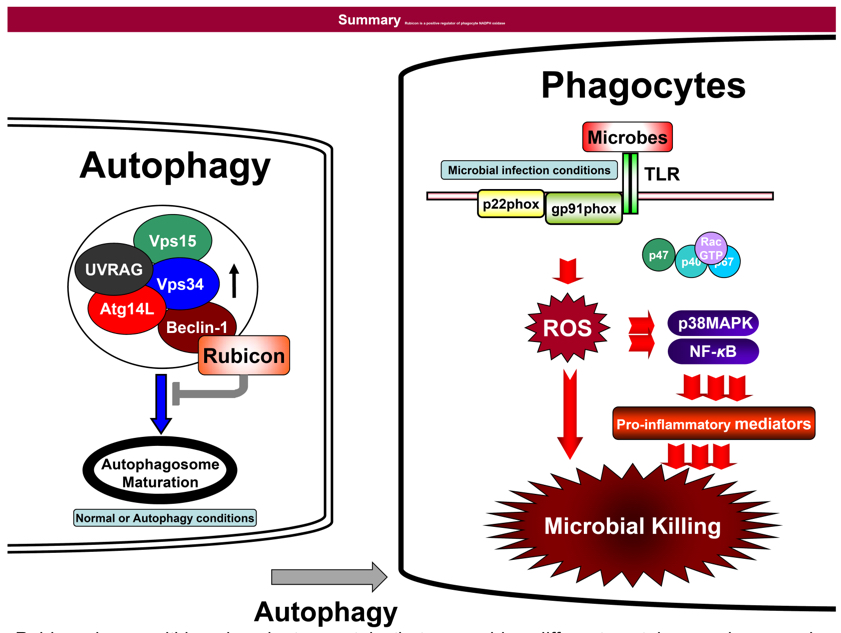
Rubicon is a multidomain adaptor protein that assembles different protein complexes under basal and PRR-activated conditions in macrophages (Cell Host & Microbe. 2012 Mar 15;11(3):264-76).
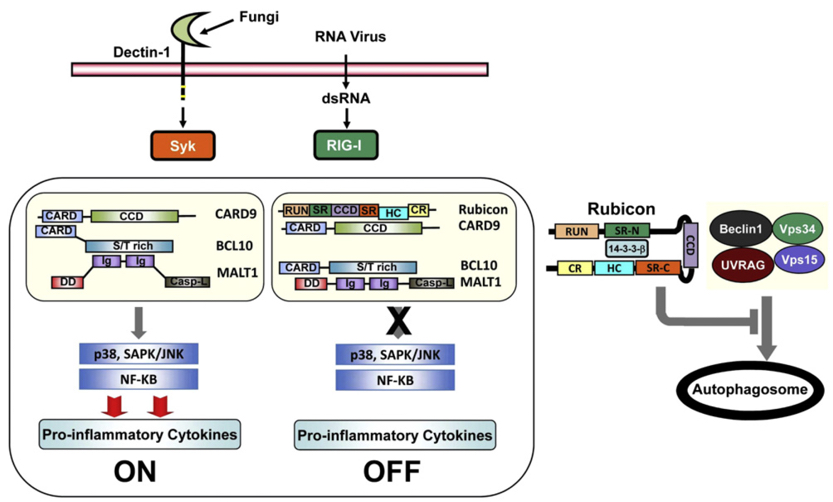
Hypothetical Model of Rubicon-Mediated Feedback Inhibition of CBM Signal Transduction (Cell Host & Microbe. 2012 Mar 15;11(3):277-89).
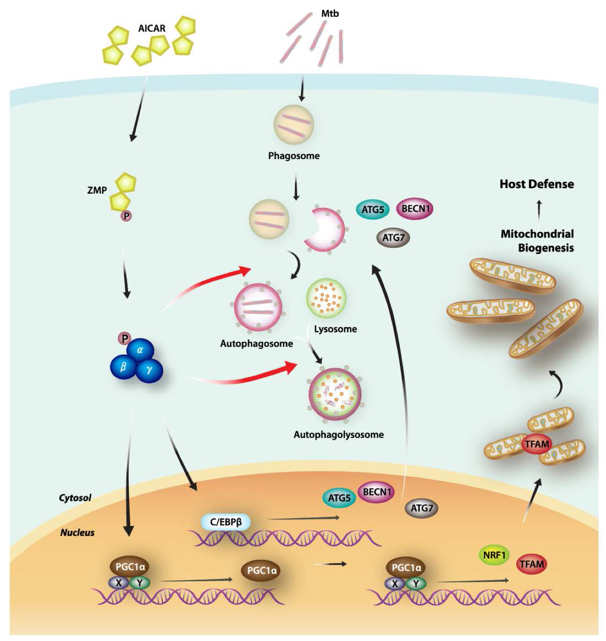
Schematic model of the mechanism by which the AMPK-PPARGC1A axis contributes to antimicrobial defense through a link between antibacterial autophagy and mitochondrial function (Autophagy. 2014 May 1;10(5):785-802).
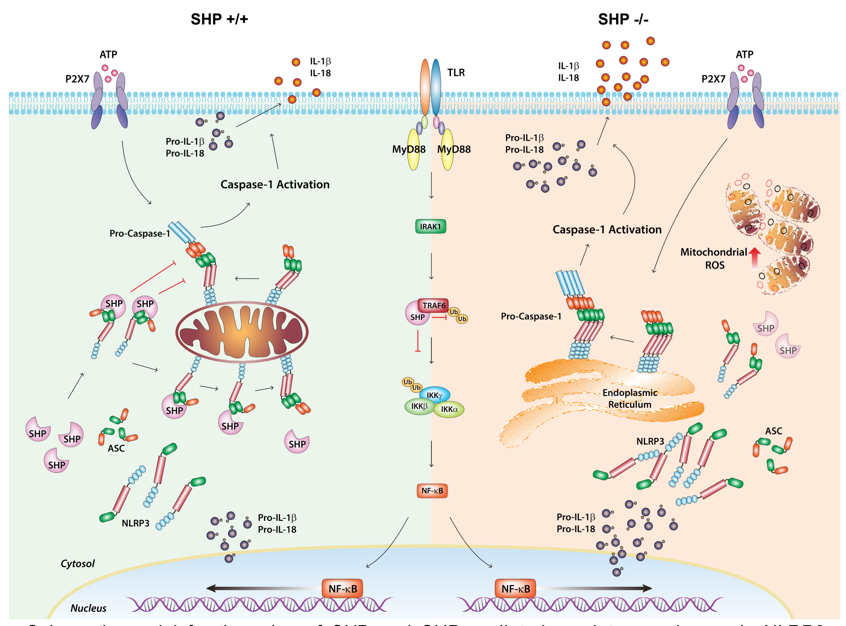
Schematic model for the roles of SHP and SHP-mediated regulatory pathways in NLRP3 inflammasome activation (Nat Commun. 2015 Feb 6;6:6115).